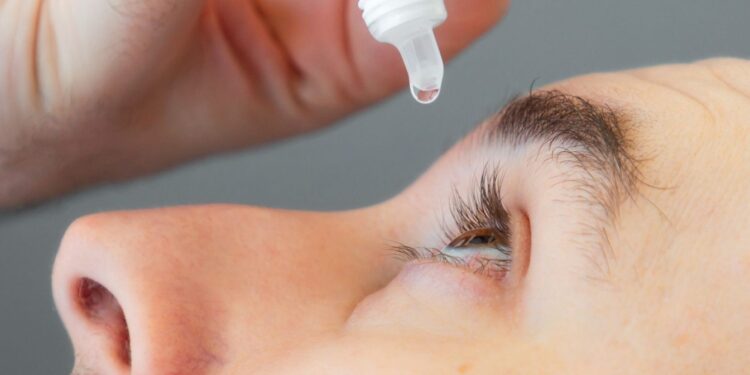
Food and Drug American Administration (FDA) has just authorized a major advance in the treatment of presbyopiathis difficulty to see closely which affects millions of people. Named Vizz, this new eye drops could well mark the end of dependence on reading glasses.
Developed by the Californian company Lenz TherapeuticsVIZZ is the first steel treatment with ACECLIDINE to receive the approval of the FDA. Its mechanism is both simple and ingenious: it slightly reduces the diameter of the pupil. This “stenopated” effect improves the development on nearby objects without affecting far vision. A single daily dose of vizz is enough to offer up to ten hours of nearly clearer vision.
Proven efficiency, without the usual disadvantages
Approval of the FDA is not the result of chance: it is based on solid clinical results. Three phase 3 trials, carried out on hundreds of volunteers, made it possible to document more than 30,000 days of treatment. These studies have shown that near vision improvements appear on average 30 minutes after instillation and maintain throughout the day.
Unlike other existing eye drops, Vizz has been designed not to stimulate the ciliary muscle, which avoids common side effects such as headache or blurred vision. In fact, no serious adverse event was reported during the tests, making Vizz a safe and effective alternative.
Availability and price
The launch of Vizz in the United States is scheduled for the end of 2025, with a distribution of samples that will start in October. The announced price will be about $ 79 per month. Although there is not yet official information about its distribution outside the United States, Lenz Therapeutics said that steps for international marketing are underway.
The arrival of Vizz represents a turning point for the management of presbyopia, offering a practical and non -invasive solution for those who seek to free themselves from the constraints of the reading glasses.
Free consultations and glasses offered for 80 Tunisian students